Serving the Great Lakes Bay Area
989-583-5675
Kybella
Kybella
Serving the Great Lakes Bay Area
Kybella
What is Kybella®?
Kybella® is an FDA-approved, prescription treatment that destroys fat cells under the chin, slimming and trimming the profile. Kybella® is used to eliminate the “double chins” that plague so many of us, dramatically improving facial appearance.
How does Kybella® Injection Work?
Kybella® is tailored to your unique chin profile. Dr. Barry will tailor a series of treatments for you base on how much fat is beneath your chin and your aesthetic goals. When injected into the fat beneath the chin, Kybella® permanently destroys fat cells. Your body’s natural metabolism then processes the fat cleared from the treatment area. Once the fat cells are gone, you’re left with a noticeable reduction in fullness under the chin. The active ingredient in Kybella® is synthetic deoxycholic acid. Deoxycholic acid is a naturally occurring molecule in the body that helps break down fat.
What Can You Expect?
Prior to treatment an office staff member may take your photo to track your results over time. Your healthcare specialist may use cold packs or local anesthesia to help make you more comfortable. The injection process may take 15-20 minutes. After treatment you may be excited to see results right away. It is important to be patient. Allow your body time to process the fat cleared from the treatment area after each visit. Schedule your next appointment before you leave the office. To reach your aesthetic goals, it is important to stick to your healthcare specialists tailored plans.
Improvement in self-perception after treatment
Adults treated with Kybella® reported greater improvement than patients treated with placebo when asked how they felt in these key areas:
· Happy · Bothered · Self-Conscious · Embarrassed · Looking Older · Looking Overweight
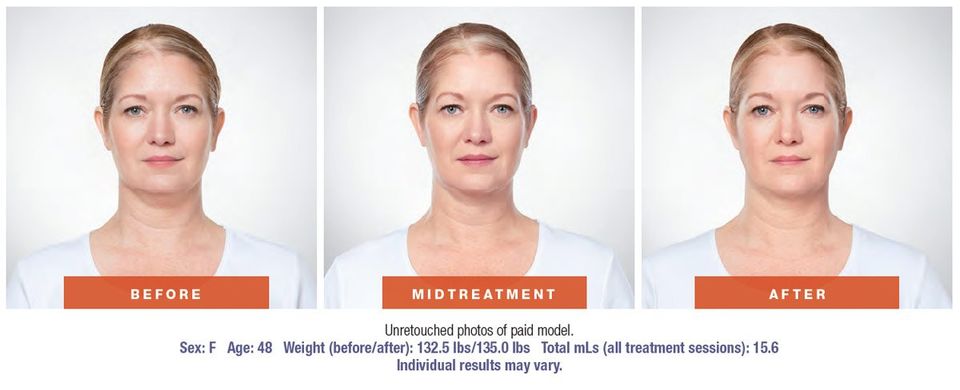
KYBELLA® (deoxycholic acid) injection 10 mg/mL Before-and-After Photos (Lisa V. 2)
Results are represented over the course of treatment; not all treatments are shown. Up to 6 treatments may be administered, spaced no less than 1 month apart.1 In clinical trials, 59% of people treated with KYBELLA® received all 6 treatments.1
Approved Use
What is KYBELLA®?
KYBELLA® is a prescription medicine used in adults to improve the appearance and profile of moderate to severe fat below the chin (submental fat), also called “double chin.” It is not known if KYBELLA® is safe and effective for use outside of the submental area or in children less than 18 years of age.
Important Safety Information
Who should not receive KYBELLA®?
You should not receive KYBELLA® if you have an infection in the treatment area.
Before receiving KYBELLA®, tell your healthcare provider about all of your medical conditions, including if you:
Have had or plan to have surgery on your face, neck, or chin; have had cosmetic treatments on your face, neck, or chin; have had or have medical conditions in or near the neck area; have had or have trouble swallowing; have bleeding problems; are pregnant or plan to become pregnant (it is not known if KYBELLA® will harm your unborn baby); are breastfeeding or plan to breastfeed (it is not known if KYBELLA® passes into your breast milk; talk to your healthcare provider about the best way to feed your baby if you receive KYBELLA®).
Tell your healthcare provider about all the medicines you take, including prescription and over-the-counter medicines, vitamins, and herbal supplements. Especially tell your healthcare provider if you take a medicine that prevents the clotting of your blood (antiplatelet or anticoagulant medicine).
What are the possible side effects of KYBELLA®?
KYBELLA® can cause serious side effects, including nerve injury in the jaw (which can cause an uneven smile or facial muscle weakness) and trouble swallowing.
The most common side effects of KYBELLA® include swelling, bruising, pain, numbness, redness, and areas of hardness in the treatment area. These are not all of the possible side effects of KYBELLA®. Call your healthcare provider for medical advice about side effects.
Ask your healthcare provider or visit MyKybella.com for full prescribing information.
Reference: 1.
KYBELLA® Prescribing Information, April 2015.

© 2016 Allergan. All rights reserved.
All trademarks are the property of their respective owners.
MyKybella.com APC88DY16 160788
Indication
KYBELLA® (deoxycholic acid) injection is indicated for improvement in the appearance of moderate to severe convexity or fullness associated with submental fat in adults.
The safe and effective use of KYBELLA® for the treatment of subcutaneous fat outside the submental region has not been established and is not recommended.
Important Safety Information
Contraindications
KYBELLA® is contraindicated in the presence of infection at the injection sites.
Warnings and Precautions
Marginal Mandibular Nerve Injury
Cases of marginal mandibular nerve injury, manifested as an asymmetric smile or facial muscle weakness, were reported in 4% of subjects in the clinical trials; all cases resolved spontaneously (range 1-298 days, median 44 days). KYBELLA® should not be injected into or in close proximity to the marginal mandibular branch of the facial nerve.
Dysphagia
Dysphagia occurred in 2% of subjects in the clinical trials in the setting of administration-site reactions, eg, pain, swelling, and induration of the submental area; all cases of dysphagia resolved spontaneously (range 1-81 days, median 3 days). Avoid use of KYBELLA® in patients with current or prior history of dysphagia as treatment may exacerbate the condition.
Injection-Site Hematoma/Bruising
In clinical trials, 72% of subjects treated with KYBELLA® experienced hematoma/bruising. KYBELLA® should be used with caution in patients with bleeding abnormalities or who are currently being treated with antiplatelet or anticoagulant therapy as excessive bleeding or bruising in the treatment area may occur.
Risk of Injecting Into or in Proximity to Vulnerable Anatomic Structures
To avoid the potential of tissue damage, KYBELLA® should not be injected into or in close proximity (1 cm-1.5 cm) to salivary glands, lymph nodes, and muscles.
Adverse Reactions
The most commonly reported adverse reactions in the pivotal clinical trials were: injection site edema/swelling, hematoma/bruising, pain, numbness, erythema, and induration.
Whether you have a moderate amount of fat under your chin—or a bit more—ask Dr. Ronald C. Barry about nonsurgical Kybella® today!
CONTACT/LOCATION
Ronald C. Barry MD, FACS
4677 Towne Centre Road Medical Art III, Suite 105
Saginaw, MI 48604
Phone: 989-583-5675
Fax: 989-583-1939
Office Hours
Monday–Thursday: 8:00 a.m.–5 p.m.

PAYMENT OPTIONS
Patient Financing
Insurances Accepted:
AETNA
ASR
BLUE CARE NETWORK
BLUE CROSS BLUE SHIELD
COFINITY
HEALTH CARE ALLIANCE POOL (HAP)
HUMANA
MEDICARE
MEDICARE PLUS BLUE
PRIORITY HEALTH
Smarthealth
TRI CARE
Content, including images, displayed on this website is protected by copyright laws. Downloading, republication, retransmission or reproduction of content on this website is strictly prohibited. Terms of Use
| Privacy Policy



